IASO Bio Presented Study Findings on the Impact of CAR T-Cell Persistence on Clinical Outcomes in Relapsed/Refractory Multiple Myeloma with Equecabtagene Autoleucel(FUCASO) Myeloma at 2024 ASH
SHANGHAI and NANJING,China and SAN JOSE,Calif.,Dec. 10,2024 -- IASO Biotherapeutics ("IASO Bio"),a biopharmaceutical company focused on the discovery,development,manufacturing,and commercialization of innovative cell therapies and biologics,today shared findings via a poster presentation of the results on the impact of CAR T-Cell Persistence on Clinical Outcomes in Relapsed/Refractory Multiple Myeloma(R/RMM) with Equecabtagene Autoleucel (Eque-cel,FUCASO) at the 66th American Society of Hematology (ASH) Annual Meeting. The results highlighted the importance of the efficacy-to-target ratio in the treatment of multiple myeloma,emphasizing the crucial role of sustained CAR T-cell persistence,unaffected by baseline soluble B-cell maturation antigen (sBCMA) levels,to achieve optimal disease control.
Title: Impact of CAR T-Cell Persistence on Clinical Outcomes in Relapsed/Refractory Multiple Myeloma: Insights from the Phase 2 FUMANMA-1 Study
Publication Number: 4761
Presentation Time: 6:00 PM -8:00 PM,Monday,December 9,2024(San Diego)
Conclusions
The efficacy-to-target ratio is a crucial determinant of clinical outcomes in patients treated withEque-cel,underscoring the importance of CAR T-cell persistence.
This ratio may serve as a biomarker for future treatment planning,highlighting the need for sustained CART-cell persistence to achieve optimal disease control.
BaselinesBCMA levels do not negatively affect the persistence and efficacy of Eque-cel.
This post-hoc analysis of the Phase 2 study of FUMANBA-1 primarily investigated the relationship between CAR T-cell persistence and progression-free survival (PFS) as well as time to progression (TTP). It also examined the impact of sBCMA levels through the lens of the efficacy-to-target ratio,measured by the ratio of vector copy number (VCN) duration to baseline sBCMA levels. With a median follow-up of 24.67 months,107 patients in 14 centers were treated with Eque-cel.
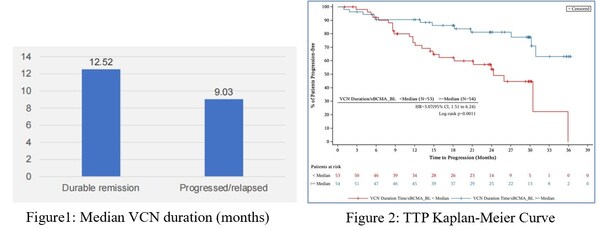
At the data cutoff,the ongoing remission group showed a longer median VCN duration than those with progression or relapse(12.52m vs 9.03m),as shown in Figure 1.The median efficacy-to- target ratio for the 107 subjects was 1.05 (days*mL/ng). As shown in Figure 2,those with a lower ratio had a significantly higher risk of disease progression,with a hazard ratio (HR) for TTP of 3.07 (95% CI: 1.51–6.24,p=0.0011) and for PFS of 2.3 (95% CI: 1.27–4.14,p=0.0045),both indicating a strong correlation between the efficacy-to-target ratio and the risk of disease progression.
Among baseline characteristics—includingECOG score,R-ISS and ISS disease stages,tumor BCMA expression,tumor burden,baseline sBCMA level,previous autologous stem cell transplantation (ASCT),triple-class exposure,bridging therapy,prior CAR-T treatment,and lymphodepleting conditioning—only previous ASCT (n=30) and ADA as post-infusion factors were significantly associated with Eque-cel persistence,with hazard ratios (HR) 0.35 and 5.79,respectively,as shown in Table 1.
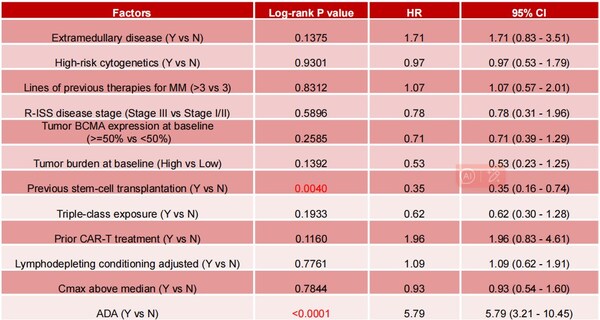
Table 1:Influencing factors analysis for CAR-T cell persistence
Among the 107 subjects,only 14 exhibited an aplastic neutrophil recovery phenotype,characterized by persistent neutrophil counts below 500/μl for 14days or more. This phenotype did not significantly affect VCN duration compared to patients without the condition,indicating that the long-term persistence of Eque-celdoes not increase hematological toxicity.
The principal investigator of this study, Professor Lugui Qiu,from the Institute of Hematology and Blood Diseases Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,and Professor Chunrui Li,from Tongji Hospital,Tongji Medical College,Huazhong University of Science & Technology,stated:
"This post-hoc analysis explored the determinants of long-term efficacy after CAR-T treatment through the lens of the efficacy-to-target ratio (VCN persistence/sBCMA). It confirmed the significance of this ratio in achieving long-term control of multiple myeloma (PFS and TTP). The findings suggest that a CAR-T product with long-lasting efficacy,unaffected by the target,which means not affected by sBCMA levels,would be a more optimal choice in the treatment of MM.
The result further highlighted the crucial role of sustained persistence of CAR-T cells in improving the prognosis of patients with R/RMM. In our 2023 ASH oral report,we previously confirmed a positive correlation between Eque-cel persistence and the maintenance of MRD negativity. The median duration time of CAR-T cells for Eque-cel was 419 days,which may be a key factor in achieving the high MRD negativity rate of 97.8% and the sustained MRD negativity rate of 81.7% at 12 months post-infusion.
Notably,only 14 of 107 subjects exhibited aplastic neutrophil recovery lasting 14 days or more. This finding indicates that the long-term persistence of Eque-cel does not increase hematological toxicity,further validating the safety of this therapy."
Dr. Jie Chen,Chief Medical Officer of IASO Bio,stated:
The outcomes presented by IASO Bio at the ASH Annual Meeting highlighted the significant impact of the efficacy-to-target ratio on disease control in multiple myeloma. The data demonstrated that subjects with an efficacy-to-target ratio above the median value had a better prognosis in terms of PFS and TTP. These findings further confirmed the positive effect of long-term CAR-T cells persistence in achieving sustained remission. Eque-cel was deliberately selected in its early development to be a candidate molecule not influenced by sBCMA levels,offering patients deeper and more durable remission. Additionally,the study showed that only a small number of patients experienced neutropenia lasting more than 14 days after treatment with Eque-cel. The result affirmed that the long-term persistence of Eque-cel does not increase the hematological toxicity,further validating its safety profile.
We are fully confident in the outstanding efficacy and safety of Eque-cel. Currently,We have initiated and are rapidly advancing a randomized controlled Phase 3 clinical study of this therapy for second- and third-line multiple myeloma. We look forward to bringing this advanced therapy to a broader population of multiple myeloma patients both domestically and internationally as soon as possible."
About FUMANBA-1 Study
FUMANBA-1 is a single-arm,open-label phase 1b/2 registrational clinical study conducted in 14 Chinese centers to assess the efficacy and safety of the Equecabtagene Autoleucel in patients with R/RMM who have received ≥3 lines of prior therapies. The trial enrolled patients with RRMM who had previously received at least three lines of therapy,including proteasome inhibitors and immunomodulatory agents-based chemotherapy regimens,and had disease progression on their last line of therapy. Patients who had previously received BCMA CAR-T therapy were also eligible for enrollment.
About IASO Bio
IASO Bio is a biopharmaceutical company focused on the discovery and development of innovative cell therapies and biologics for oncology and autoimmune diseases. IASO Bio possesses comprehensive capabilities spanning the entire drug development process,from early discovery to clinical development,regulatory approval,and commercialization.
Its pipeline includes a diversified portfolio of over 10 novel products,including Equecabtagene Autoleucel (a fully human BCMA CAR-T injection). Equecabtagene Autoleucel received Biologics License Application (BLA) approval from China's National Medical Products Administration (NMPA) in June 2023 and U.S. FDA IND approval for the treatment of R/RMM in December 2022.
Leveraging its strong management team,innovative product pipeline,as well as integrated and high quality manufactural and clinical capabilities,IASO aims to deliver transformative,curable,and affordable therapies that fulfil unmet medical needs to patients in China and around the world. For more information,please visit http://www.iasobio.com or www.linkedin.com/company/iasobiotherapeutics.
